Alternative Cancer Treatments
Natural Healing For Cancer Patients
Featured Cancer-Fighting Therapies
Read More
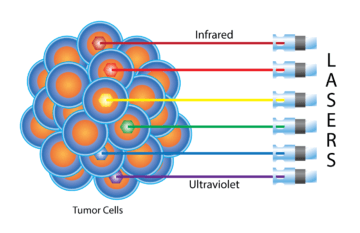
Photo Dynamic Therapy Plus
An innovative and effective treatment with a long list of benefits, Photo Dynamic Therapy Plus builds on the foundation of Sono-Photo Dynamic Therapy with a more direct application of low-level laser light.
Read More
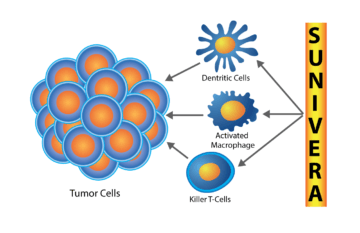
Sunivera™ Bio-Immunotherapy Protocol
Combining a powerful, immunotherapeutic macrophage activating factor (MAF) with synergistic therapies and nutraceuticals that work together to modulate the innate and adaptive immune systems.
Read More
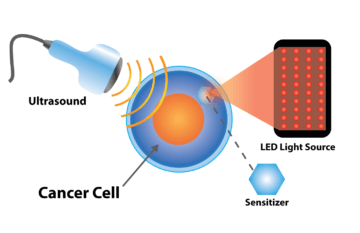
Sono-Photo Dynamic Therapy
Using non-toxic sensitizers that selectively concentrate in cancer cells, these can be activated using predetermined sound (Sono-Dynamic Therapy) and light (Photo-Dynamic Therapy) frequencies.
Regardless of your situation, we believe there is HOPE!
Let’s Talk
We understand that you may have many concerns right now.
Our admissions counselors are happy to help you find the answers you need.
Get a FREE personalized treatment plan today.
What To Expect
Fill out a secure and confidential form so we can get to know you a little better before the call.
– Less than 5 min to complete.
One of our admissions counselors will call you quickly to lend support and answer any questions that you may have about Hope4Cancer.
 Light and Sound Therapies
Light and Sound Therapies Bio-Immunotherapies
Bio-Immunotherapies Thermal Energy Therapies
Thermal Energy Therapies Biological IV Therapies
Biological IV Therapies  Metabolic Therapies
Metabolic Therapies Oxygen Therapies
Oxygen Therapies Anti-Microbial Therapies
Anti-Microbial Therapies Anti-Angiogenic Therapies
Anti-Angiogenic Therapies Anti-Inflammatories
Anti-Inflammatories Nutritional Therapies
Nutritional Therapies Magnetic/Electrical Fields
Magnetic/Electrical Fields Detoxification Therapies
Detoxification Therapies Exercise Therapies
Exercise Therapies Emotional/Spiritual
Emotional/Spiritual