Summary:
- In this second installment in our ongoing series, we discuss adaptive immunity, which serves as the second line of defense following our innate immune system.
- The adaptive immune system specifically targets infected or mutated cells, maintains immunological memory, and is able to distinguish between our own cells (“self”) and external invaders (“non-self”).
- The major components of the adaptive immune system are B cells, which produce antibodies, and T cells, which stimulate adaptive immunity by directly interacting with non-self antigens presented by infected or abnormal cells.
Introduction
Immunomodulation is one of the 7 Key Principles of Cancer Therapy and incorporated into every Hope4Cancer treatment protocol. In this second in a series of articles on this topic, we hope to unravel some of the mysteries of the complex adaptive immune system that protects us every day. We will navigate you through some of its key concepts and clarify some of the understandably confusing terminology. If you haven’t already, I would encourage you to read my article on the innate immune system first, so that you can become familiar with some of the terminology we discussed there and will use in this article.
A common oversimplified understanding of the adaptive immune response is the mental picture of antibodies floating around that attack invading pathogens. While this is partially true, like anything in the human body, the story is a bit more complicated. But, that’s where the beauty of the immune system lies – the simplicity in its complexity. In the previous article in this series, we discussed the properties of the innate immune system and the broad-spectrum approach it takes to killing pathogens—if it seems foreign, it’s a target. The innate immune system is incredibly important as our first line of defense; but, were it our only source of protection, the needed mass release of pro-inflammatory cytokines by its cells would certainly have negative effects on the body. As such, our bodies have developed a secondary layer of defense—a sophisticated and nuanced way to recognize and target pathogens, as well as infected and mutated cells. This is the adaptive immune system.
The innate and adaptive arms of the body’s immune system work as one cohesive unit responding to infection by foreign organisms, such as bacteria, viruses, fungi and parasites, or even inanimate toxic xenobiotics present in our environment. However, when you look closely, the innate and adaptive immune responses have very different mechanisms, both independent of and interdependent with each other. So, let us focus on how adaptive immunity works.
What is Adaptive Immunity?
Unlike the innate immune response, adaptive immunity is highly specific to one pathogen and its variants by learning how to recognize some of its fragments which are called antigens. If these antigens are present in the other variants of the pathogen as they often are, it becomes possible for the adaptive immune system to recognize a series of its variants (like the variants of the SARS-CoV-2 virus which, barring some mutations, are largely similar in composition and structure).
Adaptive immune response follows the innate immune response to take care of whatever pathogens are left after the first cleanup. In the process of destroying pathogens, innate immune cells collect pathogen fragments that are presented as antigens to adaptive immune cells—much like a sniffer dog can help find a missing person by smelling a piece of his or her clothing. Once the antigen has been recognized, adaptive immune cells can seek out and destroy anything that contains that antigen – leading to an intelligent and highly specific response.
Components of the Adaptive Immune Response
To understand how adaptive immunity works, it is important to first understand the various components that make up the adaptive immune response – namely, B cells and T cells.
T Cells
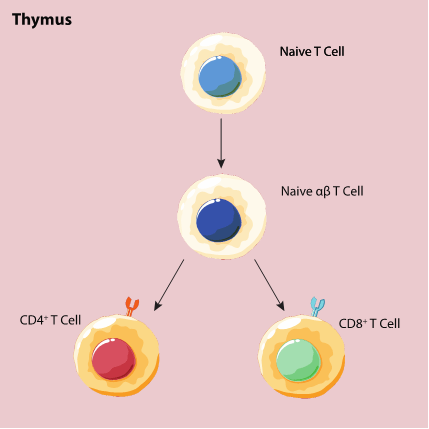
T cells develop from cells that move from the bone marrow, which then mature and develop in the thymus—hence the letter “T” in T cells [1-3]. The thymus is a small organ that is located just above the heart behind the sternum. The activity of T cells rises from the presence of receptors on their surface called T cell receptors (TCR). Through a complicated process involving the random shuffling around within the “malleable” TCR gene, each TCR becomes a unique receptor tuned to recognize its target antigen through a specific adaptive immune response [4].
As T cells develop, they are first sorted into two categories: alpha-beta ( ) and gamma-delta ( ) [4]. You may remember T cells from my previous article as a nonspecific but integral part of our innate immune system. T cells differentiate into subtypes of T cells that engage in adaptive immunity.
There are three common types of T cells that circulate throughout the body: CD4-positive (CD4+) T cells, CD8-positive (CD8+) T cells, and regulatory T cells (Tregs). Figure 1 illustrates how T cells develop within the thymus.
CD4+ T Cells
Before we discuss what CD4+ T cells are able to do, you’re probably wondering, “What does CD4+ mean?” Like the TCR, there are all kinds of receptors and proteins on the cell surface. CD4 is a surface receptor protein that is present on several immune cells including monocytes, macrophages, dendritic cells and, of course, T cells. CD4+ just means that we are dealing with T cells that have the CD4 receptor on their cell surface. CD4 is a co-receptor with the TCR that helps to bind CD4+ T cells to cells that present the antigen (called “antigen-presenting cells” or APCs) via a cell receptor called MHC-II. An example of an APC is a dendritic cell, that specializing in transferring antigen information from the innate to the adaptive immune system.
CD4+ T cells are also called “helper T cells” due to their role in activating CD8+ T cells and B cells (both of which we’ll discuss later) to actively respond to an infection [5]. That being said, there are two types of CD4+ T cells: type 1 (Th1) and type 2 (Th2), which have very different functions from one another.
Figure 1: T cell maturation and development in the thymus.
Th1 Cells
Th1 cells help to actively respond to infections by inducing inflammatory responses against pathogens [6]. These cells release pro-inflammatory cytokines that kill the pathogen. However, because the adaptive immune system is meant to be incredibly precise in its killing effect, there needs to be a counterbalance to curb any potentially excessive and dangerous levels of inflammation. Chronic, or prolonged, inflammation can be highly dangerous, keeping the immune system in constant attack mode and potentially leading to heart disease, diabetes, cancer, arthritis and bowel diseases [7]. This counterbalance comes in the form of Th2 cells.
Th2 Cells
Th2 cells release cytokines that induce anti-inflammatory responses [6]. You can think of Th2 cells like the cleaning crew after the Th1 cells do the dirty work. In a healthy person, Th1 and Th2 responses should be in balance, allowing for a net normal with regards to inflammation. Just like too much inflammation is a bad thing, a lack of inflammation can also be detrimental. Inflammation and inflammatory response by cells are necessary to kill pathogens and infected cells, as well as to repair injuries [7]. The body is all about balance – what we know as the Goldilocks principle of getting it “just right.”
CD8+ T Cells
CD8+ T cells are the body’s assassins, actively killing invading pathogens, as well as infected or mutated cells. Also called cytotoxic T lymphocytes (CTLs), CD8+ T cells contain the surface receptor CD8 which, when co-stimulated with the TCR, allows the cells to release inflammatory cytokines and phagocytose to engulf and digest the pathogens [8]. The term “cytotoxic” literally means “able to kill cells,” a term that gives a clear indication of these cells’ functions. These cells receive antigens from innate immune cells or from helper T cells via the MHC-I receptor and then go on the hunt for pathogens or cells that express these antigens [9].
CTLs release particles called granules to induce apoptosis – or programmed cell death – and release cytokines to induce inflammation and recruit other immune cells [9]. These granules contain perforin, which punch holes in the surface of the infected cell, and granzymes, which break up foreign proteins [10].
Just as is the case with the balance between Th1 and Th2 cells, there is a need to balance the pro-inflammatory effect of CTLs by using the anti-inflammatory function of Th2 cells. In the end, an effective immune response is all about balance or, in other words, immunomodulation.
B Cells
When I say immune system, one of the first words that probably comes to mind is “antibodies”. As we’ve seen over the past two articles in this series, there is a lot to the immune system besides antibodies. But now, we get to discover where antibodies come from. B cells develop from stem cells in the bone marrow (hence, B cell) and mature in the lymph nodes – small kidney-shaped organs spread throughout the body – and in the spleen [11]. B cells are best known for their ability to produced specialized antibodies that recognize specific antigens and allow for the killing of certain pathogens. However, there are also natural antibodies that can bind a wide array of pathogens without any honed specificity, serving a seminal role in innate immunity [12].
Antibodies
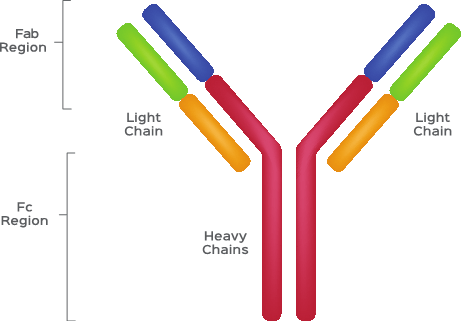
Before we jump into how B cells grow and talk about the different kinds of B cells that make up our immune systems, let’s first understand what antibodies are. The first step in understanding antibodies is knowing how they are built. You can refer to Figure 2 to follow along and visualize what an antibody looks like.
Antibodies are small, Y-shaped proteins comprised of four polypeptide chains [13]. (Just a little basic biochemistry for you – all proteins are made up of amino acids. Peptides refer to a structure of two or more amino acids. “Poly” means many. So, polypeptides are just large strings of peptides, which then make up proteins.) These four polypeptides are two identical heavy chains and two identical light chains [13].
These chains can then be broken into two regions, the fragment crystallizable (Fc) domain and fragment antigen-binding (Fab) domain [13]. The Fc domain makes up most of the antibody and largely works to recruit effector cells that can eat or kill pathogens upon binding of the Fab domain to an antigen [14-15]. The Fab domain is where the magic happens. Just as with the TCR, the gene that encodes the Fab domain goes through random assortments and, as such, develops high specificity for antigens that are presented to B cells [16].
Once a B cell is activated in the vicinity of a pathogen, it starts releasing large amounts of antibodies into the bloodstream. These antibodies bind to whatever antigen they are specific to at the Fab domain and coat, or opsonize, the pathogen. The Fc domain of the antibody then recruits cells like macrophages and natural killer cells to come in, bind to the antibody, and eat the pathogen [14-15].
Figure 2: A Cartoon Schematic of Antibody Structure
Activation of B Cells
Before B cells can generate and secrete antibodies to fight the good fight, they have to be activated by some sort of specific stimulus in order to prevent mass release of unnecessary antibodies. B cell activation is often stimulated by helper T cells and other APCs via a similar mechanism to the activation of CD8+ T cells. However, they are also able to be stimulated independent of antigen presentation from an APC.
APC-Dependent Activation
After helper T cells consume and break a pathogen down into antigens, they can present that antigen to immature B cells. After being exposed to the antigen, B cells rapidly divide and begin to develop antibodies specific for that antigen [17-18]. Immature B cells can also receive antigens from follicular dendritic cells in lymph nodes [19].
APC-Independent Activation
There are certain pathogens that B cells are able to bind via the surface B cell receptor (BCR). Upon binding, the BCR is able to stimulate rapid B cell maturation and production of antibodies against that antigen [20].
As I mentioned earlier, after being exposed to antigens, B cells mature. This maturation results in two major types of B cells – both of which produce antibodies – called transitional B cells and plasma cells.
Transitional B Cells
Transitional B cells are smaller-sized B cells that begin to secrete antibodies [21]. These cells typically develop just after the activation of immature B cells. As mentioned before, transitional B cells are able to secrete antibodies. Transitional B cells develop in two stages: T1, when the cell has recently migrated from the bone marrow to the spleen, and T2, when the cell develops into a mature B cell [22].
Plasma Cells
Plasma cells are just B cells that have entered their most mature state. Many of them are short-lived, although some have longer lifespans in the order of months. Plasma cells are larger B cells that secrete antibodies at a very rapid rate of thousands per second [21]. They do not live for very long as a result of most cellular machinery dedicating itself to this high rate of secretion rather than other essential cell functions [21]. They usually express only a single type of antibody, but in very large quantities, which is why plasma cells are an integral part of how the body develops its specific, humoral immune response.
Conclusion
I hope that you have enjoyed this article and learned more about the various components of the adaptive immune system and the sheer power, synergy and beauty of our immune system. In the next article in this series, we will start investigating how the innate immune system is able to influence the development of cancer. Stay tuned.
References
[1] Hedrick SM. Thymus lineage commitment: a single switch. Cell. 2006;124:815-822. https://doi.org/10.1016/j.immuni.2008.02.011.
[2] Jenkinson EJ, Jenkinson WE, Rossi SW, Anderson G. The thymus and T-cell commitment: the right niche for Notch? Nat Rev Immunol. 2006;6:551-555. https://doi.org/10.1038/nri1883.
[3] Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127-135. https://doi.org/10.1038/nri1781.
[4] Bonilla FA, Oettgen HC. Adaptive immunity. J Allergy Clin Immunol. 2010;125 (2 Suppl 2):S33-40. https://doi.org/10.1016/j.jaci.2009.09.017.
[5] Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. Helper T Cells and Lymphocyte Activation. https://doi.org/10.1093/aob/mcg023.
[6] Berger A. Th1 and Th2 responses: what are they? BMJ. 2000;321:424. https://doi.org/10.1136/bmj.321.7258.424.
[7] Harvard Men’s Health Watch. Understanding acute and chronic inflammation. https://www.health.harvard.edu/staying-healthy/understanding-acute-and-chronic-inflammation.
[8] Al-Shura AN. Advanced Hematology in Integrated Cardiovascular Chinese Medicine. Massachusetts: Academic Press; 2020. 7 – Lymphocytes. https://doi.org/10.1016/B978-0-12-817572-9.00007-0.
[9] Janeway CA Jr., Travers P, Walport M, Shlomchik MJ. Immunobiology, 5th edition. New York: Garland Science; 2001. T cell-mediated cytotoxicity. Available from: https://ncbi.nlm.nih.gov/books/NBK27101/.
[10] Voskobionik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol. 2015;15:388-400. https://doi.org/10.1038/nri3839.
[11] Kondo M. Lymphoid and myeloid lineage commitment in multipotent hematopoetic progenitors. Immunol Review. 2010;238(1):37-46. https://doi.org/10.1111/j.1600-065X.2010.00963.x.
[12] Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26:347-362. https://doi.org/10.1007/s00281-004-0182-2.
[13] Chiu ML, Goulet DR, Teplyakov A, Gilliland GL. Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies (Basel). 2019;8(4):55. https://doi.org/10.3390/antib8040055.
[14] Janeway CA Jr. Travers P, Walport M, Shlomchik MJ. Immunobiology, 5th edition. New York: Garland Pub; 2001. Principles of innate and adaptive immunity. Available from: https://www.ncbi.nlm.nih.gov/books/NBK10757/.
[15] Delves PJ, Martin SJ, Burton DR, Roitt IM. Roitt’s essential immunology, Thirteenth edition. Chichester, West Sussex: John Wiley & Sons, Ltd; 2017. Available from: https://www.worldcat.org/title/roitts-essential-immunology/oclc/949912256.
[16] Market E, Papavasiliou FN. V(D)J Recombination and the Evolution of the Adaptive Immune System. PLoS Biol. 2003;1(1):e16. https://doi.org/10.1371/journal.pbio.0000016.
[17] Crotty S. A brief history of T cell help to B cells. Nat Rev Immunol. 2013;13(7):475-486. https://doi.org/10.1038/nri3803.
[18] Murphy K. Janeway’s Immunobiology (8th ed). New York: Garland Science; 2012.
[19] Janeway CA Jr., Travers P, Walport M, Shlomchik MJ. Immunobiology, 5th edition. New York: Garland Science; 2001. B-cell activation by armed helper T cells. Available from: https://ncbi.nlm.nih.gov/books/NBK27101/.
[20] Yussef M-I, Pierobon P, Reversat A, Lennon-Duménil A-M. How B cells capture, process and present antigens: a crucial role for cell polarity. Nat Rev Immunol. 2013;13(7):475-486. https://doi.org/10.1038/nri3469.
[21] Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. B Cells and Antibodies. https://doi.org/10.1093/aob/mcg023.
[22] Petro JB, Gerstein RM, Lowe J, Carter RS, Shinners N, Khan WN. Transitional Type 1 and 2 B Lymphocyte Subsets Are Differentially Responsive to Antigen Receptor Signaling. JBC. 277(50):P48009-48019. https://doi.org/10.1074/jbc.M200305200.