Summary
- In this first of a series of articles, we discuss innate immunity, our essential first line of defense against a whole range of invading pathogens and abnormal cells, including cancer cells.
- The components that make up the innate immune system include the skin, biomolecules present in tissues and the bloodstream, and some specialized cells such as monocytes, macrophages and natural killer (NK) cells.
- They all work together in harmony to protect the body against foreign invaders and provide the necessary setup for a more specialized immune response by the adaptive immune system.
Introduction
Immunomodulation is one of the 7 Key Principles of Cancer Therapy and incorporated into every Hope4Cancer treatment protocol. In this first of a series of articles, we hope to unravel some of the mysteries of the complex immune system that protects us every day. We will navigate you through some of its key concepts and clarify some of the understandably confusing terminology.
The body’s immune system can be thought of as the world’s most sophisticated army standing by to respond to an invasion. You have your frontline soldiers, who throw everything they can at the enemy with the intent of preventing serious damage that can result from persistent infection. Our second line of defense consists of more specialized soldiers who only strike when the time is right, and the enemy’s strategy has been studied sufficiently. These are the two main subdivisions present in the immune system. The frontline soldiers are responsible for the innate immune response and the specialized soldiers organize the adaptive immune response.
These two arms of the body’s immune system work as one cohesive unit responding to infection by foreign organisms, such as bacteria, viruses, fungi and parasites, or even inanimate toxic xenobiotics present in our environment. However, when you look closely, the innate and adaptive immune responses have very different mechanisms, both independent of and interdependent with each other. In this first article in the Immunomodulation series, we will focus on how innate immunity works.
What Is Innate Immunity?
Innate immunity can be seen as the body’s first line of defense against external pathogens that are intent on stealing the body’s resources. Unlike the adaptive immune response that is built upon learning how these pathogens work, the innate immune response is nonspecific, which means that it is empowered to strike quickly without needing to understand the invading pathogen. After all, “innate” means “that which exists from birth,” so these immune system components do not alter themselves with every new pathogen they encounter.
Innate immune response is crucial in rapidly taming the first infection event to a point beyond which it needs to be handled by the adaptive immune response. Not only does it do its best to kill pathogens, it also presents broken-up fragments of these pathogens to cells involved in adaptive immune response so they can learn and mount a more “intelligent” and specific response.
Components of Innate Immune Response
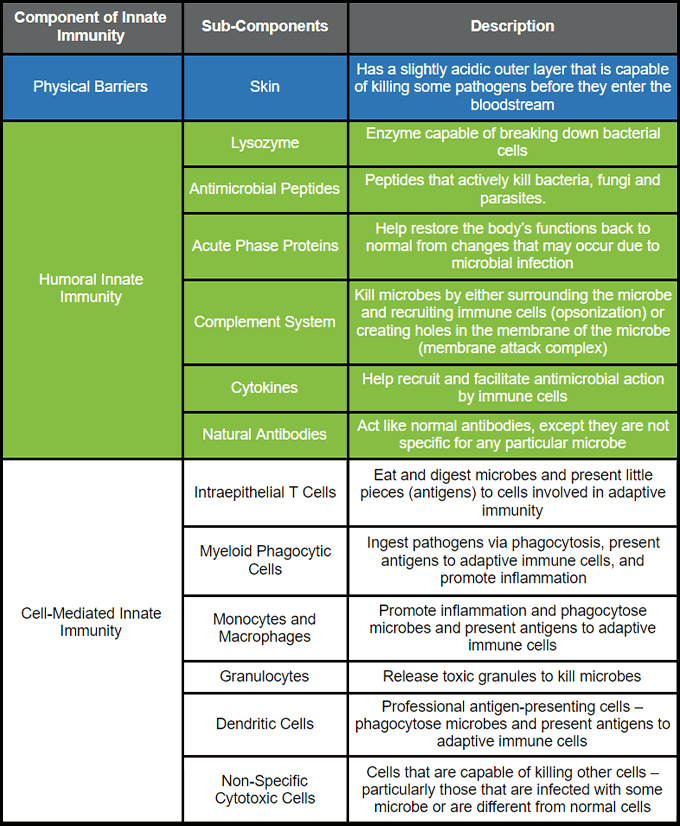
Let us first consider the three main components to innate immune response: physical barriers, humoral innate immunity, and cell-mediated innate immunity. Here is a brief summary of each component.
Physical Barriers
When we think of our skin, we think about its color, how dry it gets in the winter and summer, and how hairy it can get. But, did you know that your skin serves as one of the most important defenders of your body? It’s true!
Your skin is the biggest organ in your body and provides a layer of protection from the outside environment [1][2]. The skin has two different layers: the epidermis on the outside, made from epithelial cells, and the dermis on the inside, made from a variety of different components such as fibroblasts, blood vessels, immune system cells, and mesenchymal stem cells [1][2].
The skin as a physical barrier keeps pathogens out. The epidermis has a special “coating” layer called the stratum corneum [1][3]. This layer is weakly acidic and prevents many pathogens from infiltrating and infecting the body. The skin is also lubricated by a liquid called sebaceous fluid, which contains molecules that actively kill microbes [4][5][6][7].
Humoral Innate Immunity
The body’s immune system has mechanisms to respond to microbes that manage to get inside the body. Humoral innate immunity is one of those mechanisms. Sadly, there is nothing funny about this term; it is based on the ancient Greek principle of the body’s “humors” – bodily fluids that influence one’s health by staying in balance [8]. Any imbalance of these fluids (blood, pus, white bile and yellow bile) was thought to result in disease [8][9]. Of course, we now know that these four liquids are not solely responsible for preventing disease. Rather, components present in the blood and lymphatic fluid that influence the creation of these liquids do. Here, we’ll discuss them in some detail.
Lysozyme
What’s the best way to get rid of bacteria? Obliterate them! Obviously, not that simple! However, there are components of the innate system whose job is to do precisely that. Lysozyme is an enzyme that actively breaks down (or lyses) bacterial cells [6][10]. An enzyme is a protein that catalyzes important and specific biochemical reactions in the body. Lysozyme can be found in the fluids secreted in nasal passages, eyes and the digestive system. They are released by certain cells that are specific to innate immune response, specifically neutrophils, macrophages and Paneth cells (we’ll discuss these cells more in the Cell-Mediated Immunity section below) [11].
Antimicrobial Peptides
Antimicrobial peptides (AMPs) are peptides that are able to actively kill bacteria, fungi, and parasites [5][12][13]. There are several types of AMPs that work in different ways. These AMPs are particularly crucial in modulating the intensity of the immune response to an infection [12][13]. If the immune response is too intense, there is potential for organ damage to occur (this is the basis for autoimmune diseases). So, this modulation of immune response, particularly with regards to inflammation, is crucial. AMPs have been shown to be produced by epithelial cells and neutrophils, as well as other innate immune cells [5][11][14].
Acute Phase Proteins
Acute phase proteins (APPs) are important in responding to changes to normal body function – such as those that result from infection. APPs are responsible for both causing and reducing inflammation [15]. One of the most well-known APPs is C-reactive protein (CRP). CRP plays a major role in recognizing pathogens, surrounding them to trigger immune response, and activate the complement system [16]. As one of the leading biomarkers for inflammation, CRP is used at Hope4Cancer as an important indicator of the level of chronic, pathological inflammatory response being experienced by our cancer patients.
Complement System
The complement system is one of the most important aspects of humoral innate immunity. Complement proteins are released by cells involved in innate immunity to destroy invading pathogens [17]. There are two processes by which complement proteins help the immune system attack pathogens: opsonization and formation of the membrane attack complex [17][18].
Opsonization is a process by which these complement proteins completely surround the pathogen [19]. These proteins then attract immune cells, which collaborate to destroy the pathogen.
The membrane attack complex is a collection of complement proteins that work together to form holes in the cell walls or membranes of invading pathogens [10][18]. This forces the internal components of the pathogen, such as DNA, necessary proteins, etc., to be ejected out of the pathogen, killing them.
Cytokines
Cytokines are important proteins that are involved in both innate and adaptive immunity. They are heavily involved in triggering antimicrobial response. There are several types of cytokines involved in immune response: interferons (IFNs), interleukins (ILs), tumor necrosis factors (TNFs), and transforming growth factors (TGFs). They can both induce and discourage inflammation, activate specific biochemical pathways that promote immune response, and regulate cell growth [17][20].
Natural Antibodies
Typically, when we think of the term “antibodies,” we think of immune responses to specific pathogens based on their interaction with B cells. However, there are more generic antibodies that just float around and can react with a wide spectrum of pathogens that enters the bloodstream [21]. These are natural antibodies. These antibodies are designed to provide an initial response. Naturally, their impact will be to a lesser degree than would be expected from the more specific antibodies tailored to target pathogens by the adaptive immune response [21].
Cell-Mediated Innate Immunity
Where do the biomolecules involved in the humoral innate immune response arise from? These substances are released mainly by immune cells, described below.
Intraepithelial T Cells
We will be describing T cells more extensively in the articles on adaptive immunity. However, some T cells are able to respond directly to an infection without having to first adapt to a specific pathogen [22][23]. Called gamma-delta T cells, these T cells are able to gobble up any pathogen and present its pieces (called antigens) to other immune cells that can adapt to the pathogen to develop a more specific response [24].
Myeloid Phagocytic Cells
Myeloid cells are immune cells that are formed and differentiate in the bone marrow. They are precursors to a lot of different types of immune cells. Phagocytosis (fa-go-sy-toe-sis) is the process by which cells “eat” pathogens and other toxins. So, putting the two together, myeloid phagocytic cells are bone marrow-derived immune cells that consume and digest pathogens. These cells are able to release cytokines, lysozyme, AMPs, and other chemicals that promote inflammation [4][25][26]. They also present antigens from pathogens that have been digested to adaptive immune response cells [10][25][26].
Monocytes and Macrophages
Monocytes are white blood cells, or leukocytes, that are present in lymphatic fluid. They are known as precursors to macrophages and dendritic cells. Derived from progenitor cells in the bone marrow, monocytes aid in immune response and circulate throughout the body, drawn towards sites of inflammation [27][28].
Macrophages are very important in innate immune function. They act directly against invading pathogens and help to spur adaptive immune function. Through the release of cytokines that promote inflammation, macrophages are able to start the killing process of pathogens, as well as recruit other immune cells to the site of infection [29]. They also actively engage in phagocytosis, eating dead cells and pathogens [29]. After eating and digesting pathogens, macrophages are able to present tiny fragments of the pathogen called antigens to adaptive immune cells [29]. One of the mechanisms of action of the Sunivera™ Bio-Immunotherapy protocol is the activation of macrophages that results in downstream activation of the adaptive immune system, designed to counteract the immunosuppressive behavior observed in tumor microenvironments.
Granulocytes
Granulocytes are leukocytes that, upon recruitment to a site of infection and activation, release granules that work to kill infecting microbes [30]. These granules are small particles bound inside a sac known as a vesicle which have varying capabilities in killing microbes.
There are three types of granulocytes: neutrophils, eosinophils and basophils. Granulocytes are not able to phagocytose to the same degree as macrophages but are unique in their ability to release granules to kill microbes [31]. Granulocytes, particularly neutrophils, are intriguing in that they live for a very short time. They phagocytose microbes and, upon release of the granules, the cell dies [32]. This is because these granules, while incredibly useful for killing pathogens, can also be dangerous to human cells [32].
Dendritic Cells
Dendritic cells (DCs) are what are called ‘professional antigen-presenting cells.’ The main task of these cells is to present antigens and, as such, serve as an important bridge between the innate and adaptive immune system [33]. DCs are known for their characteristic shape with branch-like ‘arms’ extending from the center of the cell [33]. Typically present in tissues that are in contact with the external environment, DCs travel to the lymph nodes where they help to stimulate adaptive immune response by presenting antigens obtained by phagocytosing invading pathogens [33].
Non-Specific Cytotoxic Cells
“Cytotoxic” means toxic to cells, so cytotoxic cells are cells that are able to kill other cells. This cytotoxic mechanism is non-specific, which means that these cells can kill pathogens, like bacterial cells, as well as human cells. Natural killer (NK) cells are perhaps the most well-known example of this cell type. These cells respond to human cells that have become transformed (i.e., become abnormal, such as cancer cells) or infected by some microbe [34]. Upon detecting these altered cells, NK cells secrete cytokines to kill these cells or the microbe [35][36]. They also help in regenerating and remodeling injured tissue [35][36].
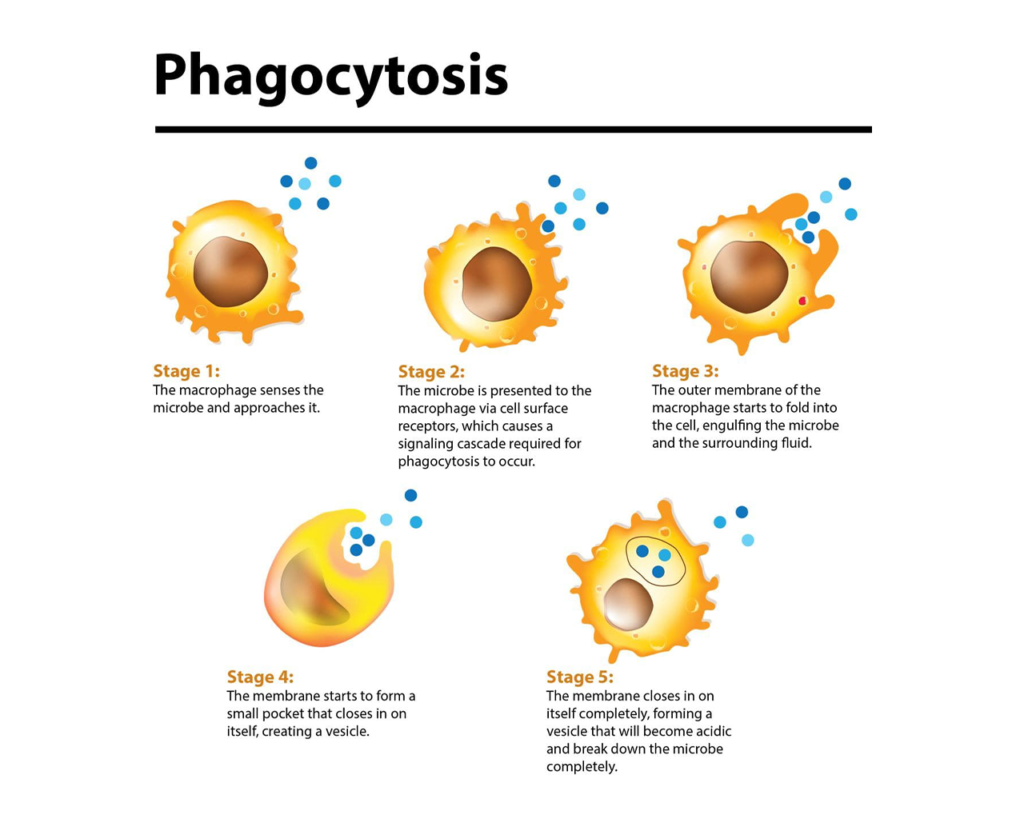
Figure 1: The Steps of Phagocytosis. The various stages of phagocytosis in a macrophage. The same processes occur in other phagocytic cells that start with detection of the microbe and engulfing it in a vesicle that allows for the cell to digest the microbe completely. Created with Biorender.com.
Conclusion
We hope this article helped you learn more about the different components of the innate immune system and how it works as a powerful, initial “filter” protecting us from pathogens and toxins. In our next article in this series, we will investigate the components and functions of the adaptive immune system, so stay tuned.
References
- Matsui T, Amagai M. Dissecting the formation, structure and barrier function of the stratum corneum. Int Immunol 2015; 27:269–80. doi: 10.1093/intimm/dxv013.
- Lillywhite HB. Water relations of tetrapod integument. J Exp Biol 2005; 209:202–26. doi: 10.1242/jeb.02007.
- Alibardi L, Toni M, Valle LD. Hard cornification in reptilian epidermis in comparison to cornification in mammalian epidermis. Exp Dermatol 2007; 16:961–76. doi: 10.1111/j.1600-0625.2007.00609.x.
- de Veer MJ, Kemp JM, Meeusen EN. The innate host defence against nematode parasites. Parasite Immunol 2007; 29:1–9. doi: 10.1111/j.1365-3024.2006.00910.x.
- Bowdish DM, Davidson DJ, Hancock RE. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr Protein Pept Sci 2005; 6:35–51. doi: 10.2174/1389203053027494.
- Akinbi HT, Epaud R, Bhatt H, Weaver TE. Bacterial killing is enhanced by expression of lysozyme in the lungs of transgenic mice. J Immunol 2000; 165:5760–6. doi: 10.4049/jimmunol.165.10.5760.
- Zasloff M. Antimicrobial peptides of multicellular organism. Nature 2002; 415:389–95. doi: 10.1007/978-981-13-3588-4_1.
- Conti AA. Historical evolution of the concept of health in Western medicine. Acta Biomed. 2018 Oct 8;89(3):352-354. doi: 10.23750/abm.v89i3.6739.
- Smith WD. The Hippocratic tradition. Ithaca: Cornell University Press; 1979.
- Abbas AK, Litchman AH, Pillai S. Cellular and molecular immunology. Philadelphia: Elsevier/Saunders; 2012.
- Tellez GA, Castano JC. Péptidos antimicrobianos. Infectio 2010; 14:55–67.
- Wilson SS, Wiens ME, Smith JG. Antiviral mechanisms of human defensins. J Mol Biol. 2013 Dec 13;425(24):4965-4980. doi: 10.1016/j.jmb.2013.09.038.
- da Silva FP, Machado MCC. The dual role of cathelicidins in systemic inflammation. Immunol Lett. 2017 Feb;182:57-60. doi: 10.1016/j.imlet.2017.01.004.
- Kahlenberg JM, Kaplan MJ. Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J Immunol. 2013 Nov 15;191(10):10.4049/jimmunol.1302005. doi: 10.4049/jimmunol.1302005.
- Gruys W, Toussaint MJM, Niewold TA, Koopmans SJ. Acute phase reaction and acute phase proteins. J Zhejiang Univ Sci B. 2005 Nov;6(11):1045-1056. doi: 10.1631/jzus.2005.B1045.
- Cray C, Zaias J, Altman NH. Acute phase response in animals: a review. Comp Med 2009; 59:517–26.
- Riera Roma M, Pérez-Martínez D, Castillo Ferrer C. Innate immunity in vertebrates: an overview. 2016 Jun;148(2):125-139. doi: 10.1111/imm.12597.
- Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res 2010; 20:34–50. doi: 10.1038/cr.2009.139.
- Bryers JD, Woodrow KA. 4.21 Engineering Interfaces for Infection Immunity. Philadelphia: Elsevier. 2017. doi: 10.1016/B978-0-08-055294-1.00264-6.
- Taylor AW. Review of the activation of TGF-beta in immunity. J Leukoc Biol 2009; 85:29–33. doi: 10.1189/jlb.0708415.
- Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol 2005; 26:347–62. doi: 10.1007/s00281-004-0182-2.
- Wu Y, Ding Y, Tanaka Y, Shen L, Wei C, Minato N et al. γδ T cells and their potential for immunotherapy. Int J Biol Sci 2014; 10:119–35.doi: 10.7150/ijbs.7823.
- Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol 2001; 2:997–1003. doi: 10.1038/ni1101-997.
- Wu Y-L, Ding Y-P, Tanaka Y, Shen L-W, Wei C-H, Minato N, et al. (2014). γδ T cells and Their Potential for Immunotherapy. Int J Biol Sci. 10(2):119-135. doi: 10.7150/ijbs.7823.
- Laskin L, Weinberger B, Laskin J. Functional heterogeneity in liver and lung macrophages. J Leukoc Biol 2001; 70:163–70. doi: 10.1189/jlb.70.2.163.
- Zenewicz LA, Shen H. Innate and adaptive immune responses to Listeria monocytogenes: a short overview. Microbes Infect 2007; 9:1208–15. doi: 10.1016/j.micinf.2007.05.008.
- Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 2006 Jan 6;311(5757):83-87. doi: 10.1126/science.1117729.
- Hettinger J, Richards DM, Hansson J, Barra MM, Joschko A-C, Krijgsveld J, et al. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol 2013 Aug;14(8):821-830. doi: 10.1038/ni.2638.
- Saldana JI. Macrophages. British Society for Immunology. Accessed February 9, 2021. https://www.immunology.org/public-information/bitesized-immunology/cells/macrophages.
- Shaz BH, Hillyer CD, Karp JK. “Blood Products.” In: Reference Module in Biomedical Sciences. New York [NY]: Elsevier. 2014. doi: 10.1016/B978-0-12-801238-3.01886-9.
- Dembic Z. “Chapter 2 – The Immune System—Definition and development of Immunity.” In: The Cytokines of the Immune System: The Role of Cytokines in Disease Related to Immune Response. Cambridge [MA]: Academic Press. 2015. doi: 10.1016/C2013-0-09724-1.
- Iba T, Hashiguchi N, Nagaoka I, Tabe Y, Murai M. Neutrophil cell death in response to infection and its relation to coagulation. 2013; 1(1):13. doi: 10.1186/2052-0492-1-13.
- Patente TA, Pinho MP, Oliviera AA, Evangelista GCM, Bergami-Santos PC, Barbuto JAM. Human Dendritic Cells: Their Heterogeneity and Clinical Application Potential in Cancer Immunotherapy. Front Immunol 2019; 9:3176. doi: 10.3389/fimmu.2018.03176.
- Caligiuri MA. Human natural killer cells. Blood 2008; 112:461–9. doi: 10.1182/blood-2007-09-077438.
- Vivier E, Spits H, Cupedo T. Interleukin-22 producing innate immune cells: new players in mucosal immunity and tissue repair. Nat Rev Immunol 2009; 9:229–34.doi: 10.1038/nri2522.
- Kakimia K, Guidottia L, Koezukab Y, Chisaria F. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med 2000; 192:921–30. doi: 10.1084/jem.192.7.921.


I have been fighting stage 4 lung cancer first chino now keytruda. After chimo there was shrinkage in the mass and a cavity , the lymph node had decreased . After 17 month the cavity has filled in with maybe fluid and lymph node increased which could be inflammation. If the drug is starting to fail can you please tell me what I can do. I eat a healthy diet of wild caught fish and a lot of vegetable and fruit. I take real mushroom extracts and about anything else to boost the immune system. Do you have any advice, I believe that I’am coming to the end of the line, started to cough and feel strange in my chest maybe a cold ‘but becoming worried. ( can you help )
Hello Paul.
We are not able to give medical advice directly through our website or social media platforms, but please contact our admissions office at 888-544-5993 or go to https://hope4cancer.com/schedule-a-call/ and fill out the form and one of our admissions officers can get you a consultation with our doctor.
Your blog related to immunity is very much helpful to know about cancer and immunomodulation. Thanks for sharing such a great information.
when will the next article in the series be posted? Thank you for such great info.
We are so happy to hear you enjoyed this! We are targeting this to be released in the next 2-3 weeks!
This is an excellent article and I felt very empowered reading it and finding out how my body works. Thanks so much!
We are so happy to hear that and thank you for the feedback and support.