In the early 1990s, researchers began exploring a novel approach to cancer treatment that utilizes the power of sound waves. This innovative technique, known as Sonodynamic Therapy (SDT), alongside its light-based counterpart, Photodynamic Therapy (PDT), represented a paradigm shift in oncology. In a world accustomed to toxic treatment approaches and invasive surgical procedures, SDT and PDT offered a far gentler, yet potentially potent alternative for cancer patients. While PDT has been extensively researched for decades, SDT has only recently started to gain attention from the scientific community, with research efforts intensifying to unlock its full potential.
At Hope4Cancer, we recognized the promise of these therapies early on, integrating them into our treatment protocols since the early 2000s, using the combined term Sono-Photo Dynamic Therapy (SPDT). Our pioneering use of SDT and PDT as integrative therapies, guided by the 7 Key Principles of Cancer Therapy®, has positioned us at the forefront of non-invasive integrative oncology. These cutting-edge modalities offer the potential for enhanced therapeutic efficacy with minimal side effects, providing newer and safer avenues to address the challenges posed by cancer, and challenging the long-held belief that effective cancer treatment must necessarily be harsh and debilitating.
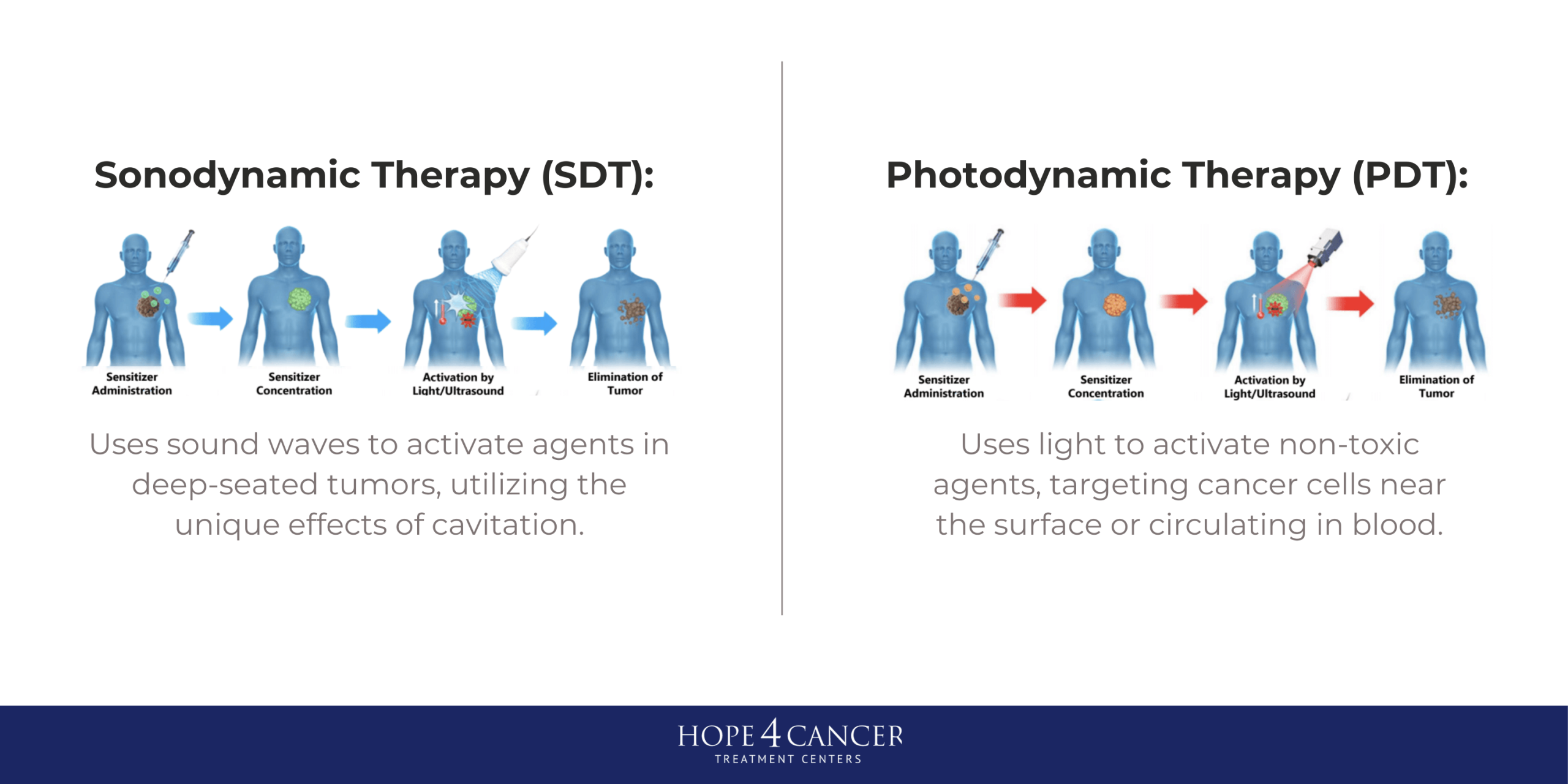
Understanding Sonodynamic Therapy (SDT)
Sonodynamic Therapy (SDT) utilizes ultrasound waves to activate sonosensitizers, which are compounds that generate reactive oxygen species (ROS) upon exposure to sound waves. The generation of ROS leads to oxidative stress in cancer cells, ultimately resulting in cell death. Both SDT and PDT have their specific advantages. SDT is particularly beneficial for treating solid tumors due to its ability to penetrate deeper tissues compared to light used in PDT, making it suitable for targeting tumors located in challenging anatomical sites such as the brain or the bone (1-3). The use of ultrasound allows for a more uniform distribution of sensitizer compounds within the tumor microenvironment, potentially overcoming limitations associated with conventional drug delivery methods (4).
Specific Advantages of Sonodynamic Therapy (SDT) and Photodynamic Therapy (PDT)
One of the combined benefits of SDT and PDT is the ability to use sensitizers that are not toxic to healthy cells in their pre-activated forms but take advantage of the enhanced metabolic rates in cancer cells to concentrate in tumor tissues. As a result, when activating light and sound are used, the treatment is very specific to tumor tissue, resulting in minimal effects to surrounding healthy tissues. Both PDT and SDT can be used long-term, unlike more conventional approaches that have more limited therapeutic windows within which they can be used. Both PDT and SDT can be administered multiple times without the same risk of resistance development, making it a more sustainable treatment option (5).
One of the primary advantages of SDT over PDT is its superior tissue penetration. While PDT relies on light, which can be significantly attenuated by biological tissues, ultrasound waves can penetrate several centimeters into the body, allowing for the treatment of deeper tumors without damaging surrounding healthy tissues (1). Additionally, for deep-seated tumors where light cannot penetrate, SDT can be performed in a non-invasive manner, reducing the need for surgical interventions and associated complications (6).
SDT has also shown to strongly reverse the acidity, hypoxia and hypermetabolic microenvironment of bone tumors, which results in the formation of a large quantity of ROS, as well as creating more oxidative conditions within the cancer cells by converting glutathione (an antioxidant) to glutathione disulfide (an oxidant). The enhanced oxidative conditions induce cancer cells to die through different mechanisms, such as ferroptosis, pyroptosis, and necroptosis (3).
PDT takes advantage of extensive research on photosensitizers as well as technological advances that allow the delivery of therapeutic light in innovative ways that range from being completely non-invasive to minimally invasive. For instance, at Hope4Cancer, we use different types of lasers and LED light sources that can be administered topically, endoscopically (laser is introduced through an endoscope to the target area), interstitially (laser is introduced directly into the tumor), intravenously, or systemically (where light is applied to the whole body).
One of the key benefits of PDT is its ability to induce localized effects because accurately delivered light sources can be precisely targeted to the tumor site, allowing for controlled activation of the sensitizers and reducing the likelihood of damage to surrounding healthy tissues (7). Furthermore, PDT has been extensively studied and clinically validated, providing a wealth of data on its safety and efficacy across various cancer types (8).
PDT also benefits from the availability of a wide range of photosensitizers, which can be selected based on the specific characteristics of the tumor and the desired treatment outcome. This versatility allows clinicians to tailor treatment plans to individual patient needs, optimizing therapeutic efficacy (8).
Mechanisms of Action
The mechanisms underlying SDT and PDT are fundamentally linked to the generation of reactive oxygen species (ROS). Figure 1 summarizes the various mechanisms of SDT. A key distinction between SDT and PDT lies in how ultrasound waves in SDT can induce cavitation phenomena, leading to the formation and collapse of microbubbles within the tumor microenvironment. There are two types of cavitation:
- Inertial cavitation: Generates bubbles that expand to almost twice their original size and burst, creating localized areas of extremely high temperatures and pressures. This process can destroy cancer cells through direct mechanical damage, as well as through enhanced ROS production.
- Stable cavitation: Provides sustained oscillation of bubbles, activating sonosensitizers. This can lead to “sonoluminescence,” a phenomenon where light is emitted, further activating sonosensitizers.
The mechanical effects of ultrasound can also disrupt tumor vasculature, improving the delivery of sensitizers and oxygen to the tumor site. These combined effects make SDT a promising approach for cancer treatment, complementing the established ROS-generating mechanisms of PDT while offering unique advantages in tissue penetration and localized damage (2, 4).

Figure 1. Mechanisms of Sonodynamic Therapy.
In contrast, PDT relies on the absorption of light by photosensitizers, which leads to the excitation of these molecules and the subsequent generation of singlet oxygen and other ROS. The localized nature of this process allows for targeted destruction of cancer cells while sparing surrounding healthy tissues (7). The choice of photosensitizer, along with the wavelength of light used, provides an advantage in guiding the therapeutic outcome, highlighting the importance of personalized treatment strategies (4).
How SDT and PDT Work alongside Bio-Immunotherapies
The synergistic relationship between SDT, PDT and bio-immunotherapies represents a sophisticated multi-modal approach to cancer treatment (Figure 2). When SDT and PDT are applied to tumor sites, they trigger immunogenic cell death, which serves as a crucial bridge between direct tumor destruction and immune system activation. This process releases vital molecular signals – DAMPs (Damage-associated molecular patterns), PAMPs (Pathogen-associated molecular patterns), and TAAs (Tumor-associated antigens) – which act as natural alarm signals for the immune system (9, 10).
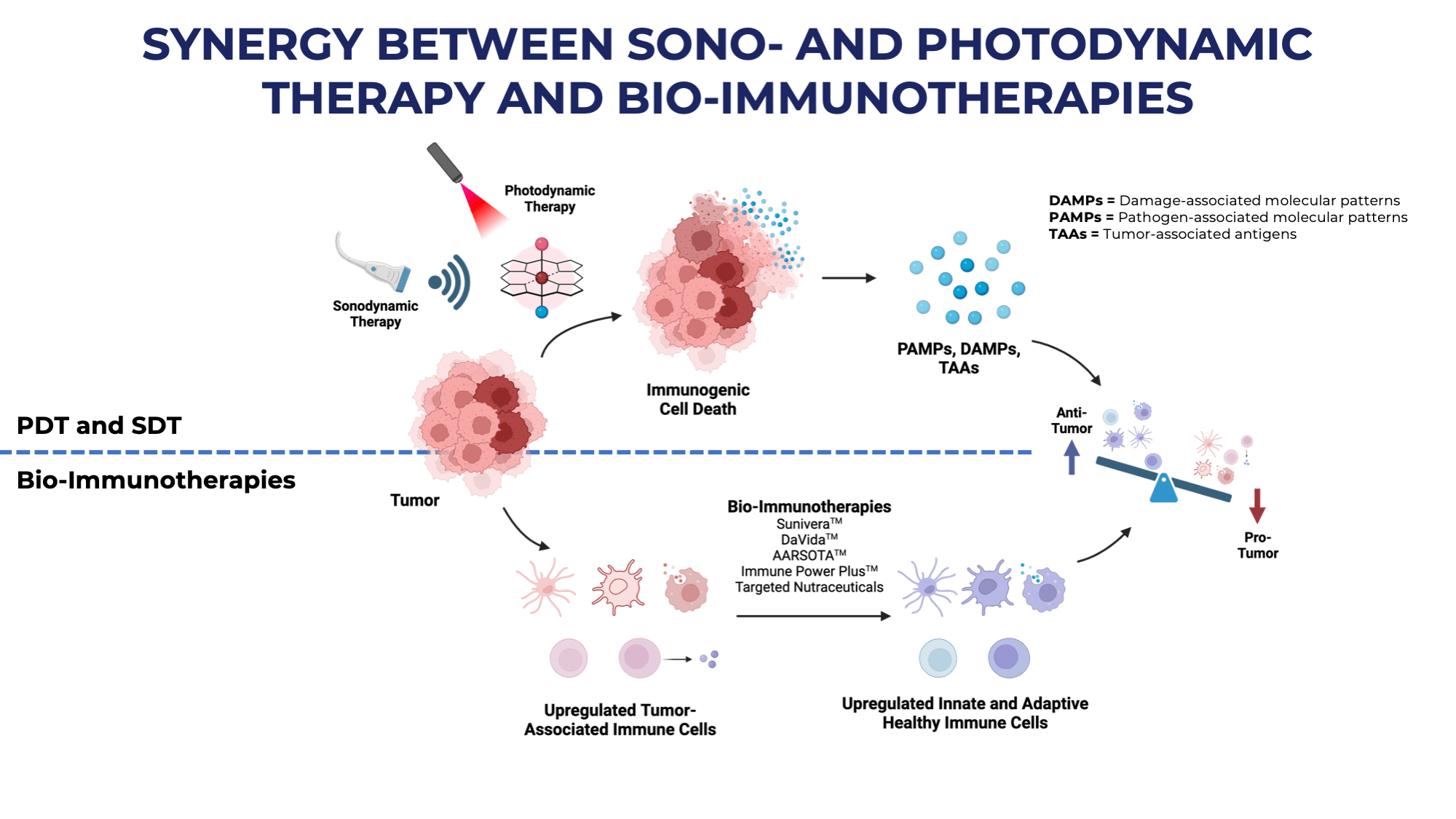
Figure 2. Synergy between SDT/PDT and Hope4Cancer’s Bio-Immunotherapies.
The combination of these therapeutic approaches works through a dual mechanism: while SDT’s ultrasound waves and PDT’s light activation directly target cancer cells, specialized bio-immunotherapies like Hope4Cancer’s Sunivera™, DaVida™, and AARSOTA™ amplify the body’s natural immune response. This approach effectively upregulates both innate and adaptive immune cells, creating a more robust defense against cancer progression.
SDT particularly excels at modifying the tumor microenvironment by reversing acidity and hypoxic conditions, while simultaneously generating reactive oxygen species (ROS). This modification creates favorable conditions for immune cell function and promotes various forms of programmed cell death.
The treatment strategy creates a favorable shift in the balance between anti-tumor and pro-tumor factors. By simultaneously attacking cancer cells through direct mechanisms while enhancing immune system function, this combined approach offers a more comprehensive and sustainable treatment option that can be administered multiple times without developing the resistance often seen in conventional therapies.
What Is Hisotripsy, and How Does It Compare to SDT?
Histotripsy is an emerging ultrasound-based technique that utilizes high-intensity focused ultrasound (HIFU) to mechanically disrupt tissue at the cellular level. This method involves delivering short bursts of ultrasound energy to create cavitation bubbles that rapidly expand and collapse, leading to the fragmentation of targeted tissues, including tumors (11). Histotripsy has shown promise in preclinical studies for the treatment of various cancers, including liver and prostate cancers, and is currently being evaluated in pre-clinical and clinical trials. The U.S. Food and Drug Administration (FDA) granted marketing authorization for HistoSonics’ Edison histotripsy system to treat liver tumors on October 9, 2023, marking the first approval of this noninvasive, ultrasound-based technology for destroying targeted liver tissue.
The precision of histotripsy allows for selective targeting of tumor tissues while minimizing damage to surrounding healthy structures. This technique can be particularly beneficial for patients with tumors that are difficult to access surgically or for those who are not candidates for traditional surgical interventions. Histotripsy is currently available only at limited locations across the United States for the specific treatment of liver tumors only, with only limited evidence of efficacy for other tumor types.
Sonodynamic therapy (SDT) offers several key advantages over histotripsy in cancer treatment, including a more targeted approach using sonosensitizers, potential systemic effects against metastases, and greater versatility across various cancer types. Unlike histotripsy’s mechanical tissue destruction, SDT’s ability to induce targeted cell death while stimulating immune responses provides distinct advantages for comprehensive cancer care, especially when combined with other treatment modalities in an integrative protocol as we do at Hope4Cancer.
Sonosensitizers: The Key to Enhanced Efficacy
Sonosensitizers play a crucial role in the success of SDT. These compounds are designed to absorb ultrasound energy and convert it into ROS, which are responsible for inducing oxidative stress and cell death in cancer cells. Recent advancements in nanotechnology have led to the development of novel sonosensitizers, including those based on piezoelectric materials that can enhance ROS production through mechanical strain induced by ultrasound (6,12). These sensitizers, that are at early stages of research, bear promise for the future enhancement of this therapeutic approach.
Conclusion
The application of sound waves in cancer treatment through Sonodynamic Therapy (SDT), represents a promising new frontier in oncology. SDT offers advantages in tissue penetration and potential to overcome drug resistance, complementing the established efficacy of Photodynamic Therapy (PDT). PDT has demonstrated its effectiveness in both localized and systemic cancer treatments, providing versatile options for various cancer types and stages. Both SDT and PDT contribute significantly to the cancer treatment toolkit, each with unique strengths. Ongoing research into new sonosensitizers and photosensitizers, along with novel ultrasound and light-based techniques, is likely to enhance cancer treatment options. This combined approach, utilizing both sound and light-based therapies, shows promise for advancing cancer care and improving patient outcomes when applied in the context of an integrative treatment protocol.
REFERENCES:
- Lin X, Song J, Chen X, et al. Ultrasound‐activated sensitizers and applications. Angewandte Chemie, 2020;59(34):14212-14233.
- Liu K, Jiang Z, Zhao F, et al. (2022). Triarylboron‐doped acenethiophenes as organic sonosensitizers for highly efficient sonodynamic therapy with low phototoxicity. Advanced Materials, 2022;34(49).
- Hou Y, Zhao D, Yang X, et al. Recent advances and pathological mechanisms in photodynamic and sonodynamic therapy in the treatment of bone tumors. Oncol Rep. 2023;50(5):198.
- Yamaguchi T, Kitahara S, Kusuda K, et al. Current landscape of sonodynamic therapy for treating cancer. Cancers, 2021;13(24), 6184.
- Sun L, Zhang J, Xu M, et al. Ultrasound microbubbles mediated sonosensitizer and antibody co-delivery for highly efficient synergistic therapy on her2-positive gastric cancer. ACS Appl Mater Interfaces, 2021;14(1), 452-463.
- Canavese G, Ancona A, Racca L, et al. Nanoparticle-assisted ultrasound: a special focus on sonodynamic therapy against cancer. Chem Eng J, 2018;340:155-172.
- Güzel E, Atmaca GY, Kuznetsov AE, et al. Ultrasound versus light: Exploring photophysicochemical and sonochemical properties of phthalocyanine-based therapeutics, theoretical study, and in vitro evaluations. ACS Applied Bio Materials. 2022;5(3):1139-1150.
- Jiang W, Liang M, Lei Q, et al. The current status of photodynamic therapy in cancer treatment. Cancers. 2023;15(3):585.
- Wang T, Peng W, Du M, et al. Immunogenic sonodynamic therapy for inducing immunogenic cell death and activating antitumor immunity. Frontiers in Oncology. 2023;13.
- Bao Y, Chen J, Huang P, Tong W. Synergistic effects of acoustics-based therapy and immunotherapy in cancer treatment. Bio Integration. 2021;2(2):61-70.
- Xu Z, Khokhlova TD, Cho CS, et al. Histotripsy: A method for mechanical tissue ablation with ultrasound. Annu Rev Biomed Eng. 2024;26:141-167.
- Li G, Wang S, Deng D, et al. Fluorinated chitosan to enhance transmucosal delivery of sonosensitizer-conjugated catalase for sonodynamic bladder cancer treatment post-intravesical instillation. ACS Nano. 2020;14(2):1586-1599.