We’ve all heard at some point that processed foods are…
Welcome To Our Blog
Education, Research, Healing & Hope
Fit to Fight: How Exercise Aids Cancer Prevention and Treatment
For a long time, conventional wisdom maintained that people with…
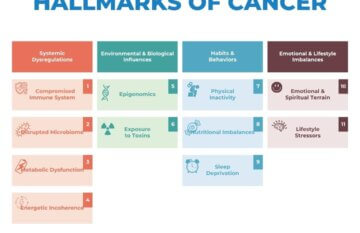
The 11 Integrative Hallmarks of Cancer
In 2000, two prominent cancer biologists, Dr. Robert Weinberg and…
Eat Smart: A Guide to the 7 Categories of Inflammatory Foods
Inflammation is the body’s natural response to injury or infection….
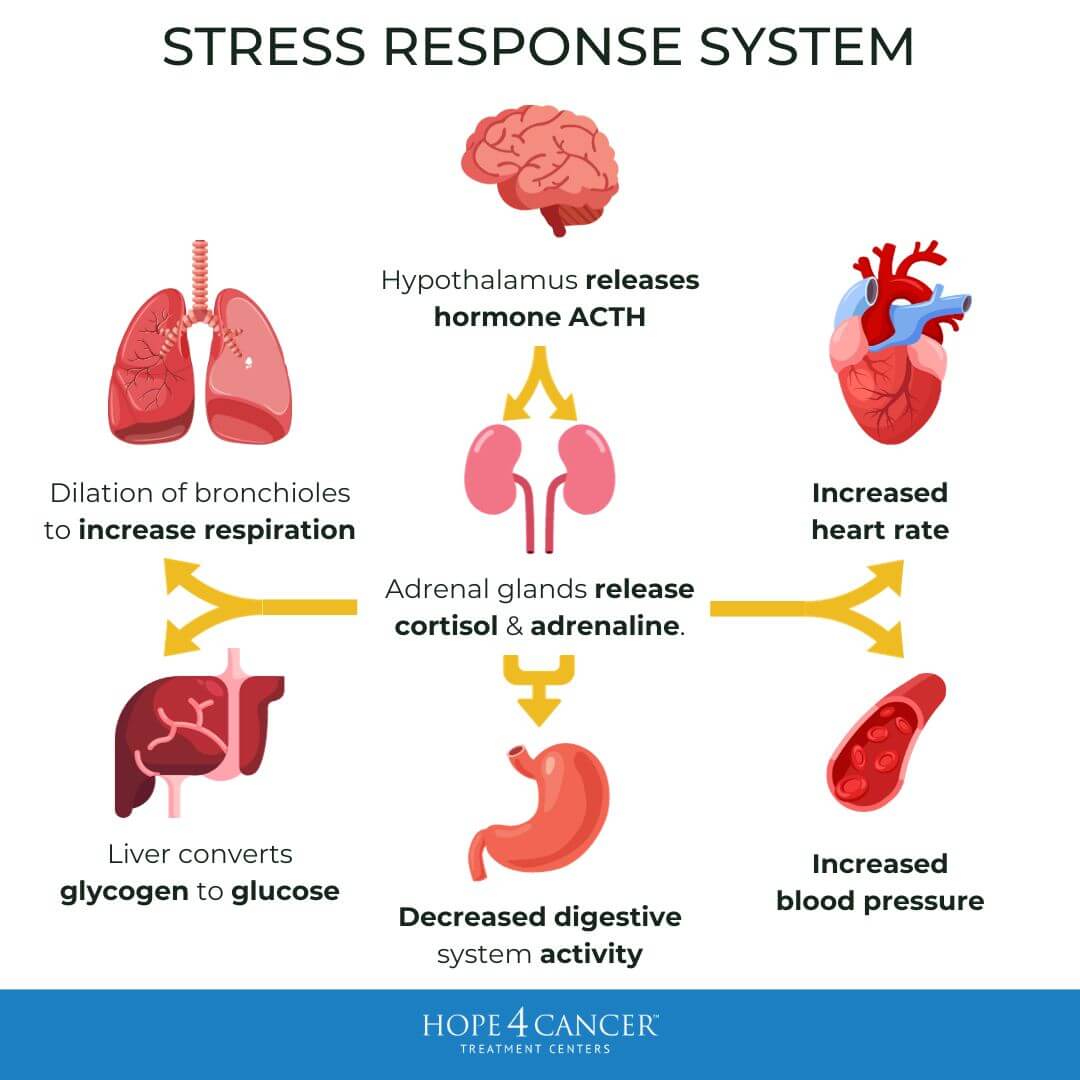
Can Stress Make You Sick?
Cancer-Causing Effects of Chronic Stress For many of us, stress…
Recipe Spotlight: Veggie Stuffed Snapper with Rustic Greens & Grains
At Hope4Cancer Treatment Centers, our dietary approach is guided by…
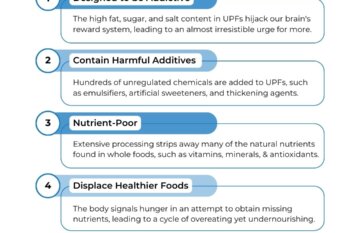
The Western Diet and Cancer: An Alarming Link
Summary The Western diet, characterized by excessive consumption of processed…
Recipe Spotlight: Portobello Breakfast
According to the American Institute for Cancer Research, about one-third…
Gastrointestinal Cancers and the Microbiome: A Close Link
The human body plays host to trillions of microorganisms living…
Recipe Breakdown: Vegan Tacos and Berry Salad
Incorporating healthy and delicious recipes into your daily routine can…